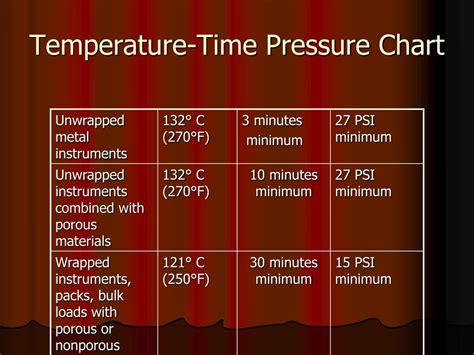
dryness test autoclave|chemiclave vs autoclave : makers The test pack should be placed horizontally in the front, bottom section of the sterilizer rack, near the door and over the drain, in an otherwise empty chamber and run at 134°C for 3.5 minutes. 813, 819 The test is used each day the vacuum-type steam sterilizer is used, before the first processed load. Air that is not removed from the chamber . $869.99
{plog:ftitle_list}
Maintaining an autoclave is essential to ensure its proper functioning and the sterilisation of equipment and materials. Regular maintenance is crucial to maintain safety and prevent contamination and .
This article provides the background and science behind the steam quality tests and proposes a risk-based approach to the routine monitoring of steam quality for a system providing steam to all pharmaceutical applications/autoclaves. Dryness testing is performed to determine the amount of moisture, or liquid . The test pack should be placed horizontally in the front, bottom section of the sterilizer rack, near the door and over the drain, in an otherwise empty chamber and run at 134°C for 3.5 minutes. 813, 819 The test is used each day the vacuum-type steam sterilizer is used, before the first processed load. Air that is not removed from the chamber .
The physical steam quality tests are strictly only applied to autoclaves, although many companies choose to perform the physical tests at other user points such as SIP systems, to get some quantifiable values for the system as a whole. .
Sterilization monitoring is necessary for each autoclave load, including mechanical and chemical indicators as required and recommended, plus, depending on your location, spore testing (biological indicators). Spore testing .This test measures the overheating of the generated steam. The temperature is measured using a temperature probe. Dryness. The dryness or saturation of steam refers to the ratio between liquid and gaseous water in the steam. For autoclaves, it is essential that the amount of liquid in the steam is limited to ensure a uniform and complete .The lifespan of an autoclave can be significantly affected by the quality of water used. To maintain an autoclave to a high standard, quality checks on the water source must also be completed per . When measured in accordance with the HTM type steam dryness test, current standards require that the steam supply should have a dryness value of .
It is generally accepted that terminally sterilized injectable articles or critical devices purporting to be sterile, when processed in the autoclave, attain a 10 –6 microbial survivor probability, i.e., assurance of less than 1 chance in 1 million that viable microorganisms are present in the sterilized article or dosage form. With heat-stable articles, the approach often is to considerably . This test exposes the autoclave’s plumbing and components to vacuum conditions and measures how much vacuum depth is lost over a given period. How do you run a vacuum leak test cycle? A typical vacuum leak test cycle consists of three vacuum/pressure pulses, followed by a 15-minute dwell time at deep vacuum. Once the vacuum leak test cycle is .The most reliable and easy-to-use steam autoclaves in the world. We're Attending Future Labs 2024! See our upcoming events. / Learn More. Chamber Blog Sales: 617-782-6071 . The good news is that essentially all laboratory and medical autoclaves on the market today can provide sterile, dry, and intact sterilization loads if provided good .How to Validate an Autoclave 2 Introduction There is an array of qualiication tests that can be conducted to validate . as a dry block, oil bath, or temperature probe. If using a dry block or an oil bath (pricing starts at . These test cycles should be .
The dry/wrapped-goods cycle was verified via a mixed load of 3 or 4 bags (enough to fill the chamber while maintaining a minimum 2-inch clearance between the load and all interior surfaces of the autoclave chamber) of dry trash and/or laundry, also sourced from the trash/laundry being generated as part of normal laboratory operations.
is autoclave dry heat sterilization

Pragmatically, if loads are not wet, the steam has a sufficiently high dryness value, though ideally, the steam should be in a dry saturated condition when it enters the sterilizer as to present the load with the absolute minimum entrained water possible and Superheat & Dryness Value Test Main Steam Supply 6 - 7 BarG Steam Header 3 - 4 BarG .The service consists of three tests: Steam Dryness, Steam Superheat and Non-Condensable Gas. These tests can be performed over the course of three days or one day. 1 | Steam Dryness Test 2 | Superheat Test 3 | Non-Condensable Gas Test. Measures the amount of moisture content in the steam. Measures the superheat of the steam.
leco hardness test blocks
If P2 –P1 20 mbar, probably the test has been performed while the chamber is moist If P3 – P2 13 mbar, the leaks that are evidently present and must be eliminated Today, most autoclaves have an automatic program for a routine tightness test, which is "analyzed" by the pressure measurement systems of the autoclave itself. Autoclave Spore Testing & Validation. Once these equipment validation activities are complete, the next step is to validate the sterilization cycle. This process, also commonly referred to as “performance qualification” or “cycle validation”, incorporates the use of biological indicators to test autoclave efficacy. Cycle validation .Thermometric test for a small load during the plateau period the temperature measured above the test pack does not exceed the temperature measured in the active chamber discharge by more than 5ºC for the first 60 s and 2ºC for the remaining period. (Ref. HTM 2010 Part 3; 13.14 (b))
It contains no added substance. The level of steam saturation or dryness and the amount of non-condensable gases are to be determined by the Pure Steam application. USP Monograph Steam Quality for Sterilization. . LEAKAGE TEST: An air leakage test for autoclave chambers is done to find out any leakage from the chamber. Air leakage test is used to establish that the quantity of air leakage into the sterilizer chamber during the . EN 285 Dryness • Dryness: excess moisture in saturated steam can cause damp loads while too little moisture cannot prevent the steam from becoming superheated during expansion into the autoclave. • Acceptance Criteria: (dryness value) »Metal Loads 0.95 »Other Loads 0.90 Ref. EN 285-2016-02 section 21.2
dry heat sterilization vs autoclave
Purpose of the Steam Dryness Value Test: To ensure that an acceptable amount of moisture is present in the steam supply. If too little moisture were present, superheating of the steam may occur. Too little moisture may prevent sterilization conditions within the load, particularly because moisture is important in breaking down the cell . In 2016, the Santa Catarina Hospital, located in the city of São Paulo, started the first evaluation of steam quality, following the indication of the technical standard ISO 17.665-1 and using the sampling techniques for detection of NCGs, steam dryness, and steam superheat described in the European standard EN 285 so that the contributions of these tests would help .
The vacuum test is an essential functional evaluation specifically designed for autoclaves equipped with vacuum cycle capabilities. This test rigorously assesses both the performance of the autoclave’s vacuum system and the integrity of its hermetic sealing. Ensuring the autoclave can achieve and maintain a sufficient vacuum is critical. Autoclave is used for sterilization of various articles in microbiology laboratory as well in sterile manufacturing. This article has procedure for autoclave validation including steam penetration, heat distribution and penetration, bio-challenge study, estimation of F0 value and acceptance criteria of steam sterilizer validation in pharmaceutical industry.
sterilize, leading to dry heat conditions. i.e. steam will not condense and provide moisture until the steam temperature has reduced to the saturation temperature. It therefore acts as hot air or dry heat. The test requires the fitting of an expansion tube onto the pitot tube used in .
An easy and reliable method to routinely test your sterilizer. ⠀ Fast Turnaround. Results available in 1 business day for steam autoclaves, 3 days for chemical, and 7 days for dry heat & EtO ⠀ Easy to use. Simply include a test strip in your next sterilization run. Return the test strip in the provided mailer ⠀ Immediate Notification Note: Steam pipe temperature within 3 ºC of Dryness Test. Dryness Value Testing According to EN 285. It’s just the ratio of moisture (liquid) in the steam (gas). . Autoclaves use pure steam to sterilize surgical instruments, laboratory equipment, and pharmaceutical containers. The high temperature and pressure ensure the destruction of all .

He demonstrated the greater power of penetration exhibited by moist heat (steam) compared to dry heat. Finally, in 1933 modern autoclave technology was introduced with the first pressure steam sterilizer that controlled performance by measuring the temperature in the chamber drain line (thermostatic trap). Prior to this date, pressure was the .The Importance of Leak Tests in Steam Sterilization The Complementary Roles of Steam Sterilizer Diagnostic Tests. Medical steam sterilizers, or autoclaves, remove air from a chamber and replace it with moist heat steam for a certain amount of time, inactivating and destroying microorganisms at a cellular level 1.. The air that needs to be removed from the chamber is a .
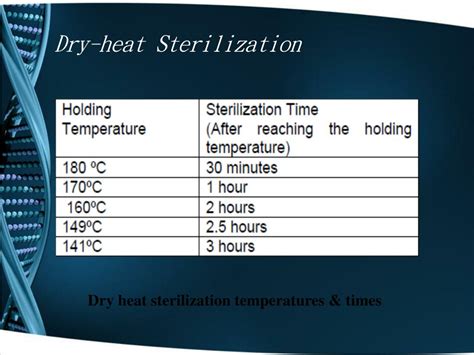
dry heat sterilization temperature chart
leco hardness tester r-60d0
leco hardness tester taking it apart
Learn how to spore test an autoclave effectively to ensure sterilization. Discover the importance of spore testing and how TOMY can help.
dryness test autoclave|chemiclave vs autoclave